

Cancer Clinical Trials Patient Guide
Mays Cancer Center researchers conduct hundreds of clinical trials to test new treatments in order to find better ways to prevent, detect and treat cancer.
Clinical trial phases
Clinical trials are studies that answer different types of research questions. Each phase of study explores a different aspect of potential new cancer services.
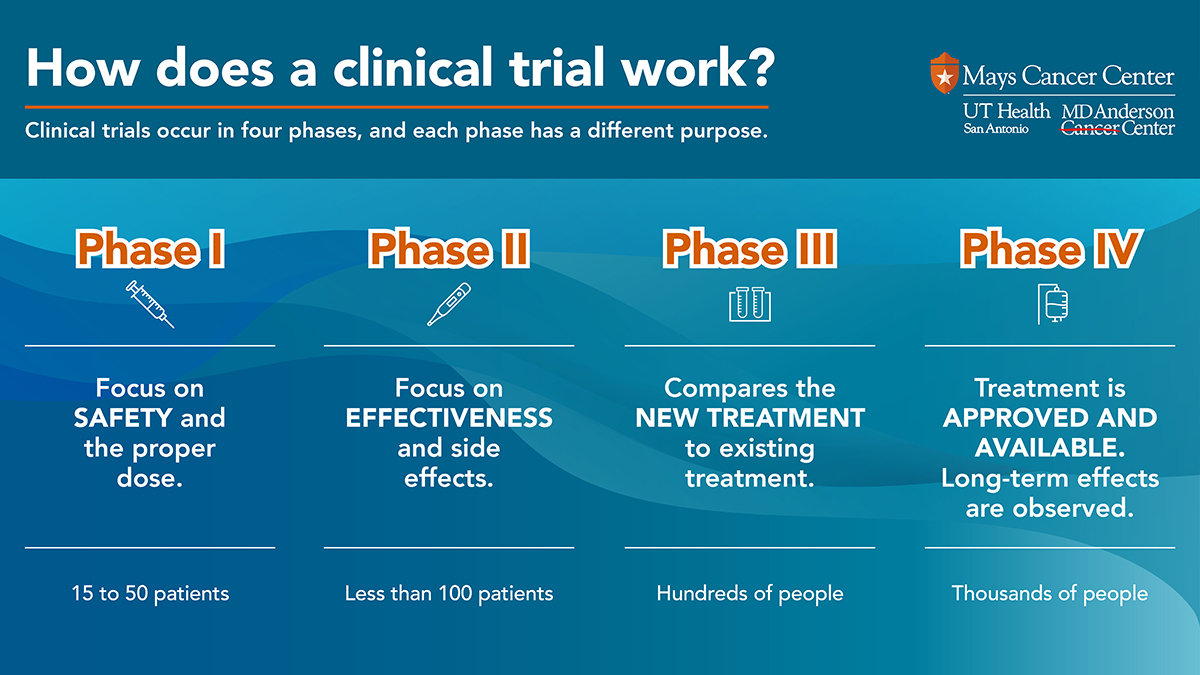
Clinical trial phases include:
- Preclinical testing: We conduct new drug studies in cancer cells and tumors growing in mice to show anti-tumor activity and safety before conducting clinical trials in cancer patients. Learn more about our Experimental and Developmental Therapeutics Program and our Cancer Development and Progression Program.
- Phase I: : First-in-human trials enable us to investigate safety and dosing in human volunteers. We study a new drug’s tolerability and anti-tumor activity in cancer patients. Find out more about our Institute for Drug Development, which hosts our phase I trials.
- Phase II: These studies take place in larger groups of cancer patients of the same cancer type.. We confirm a drug’s safety and anti-tumor activity (efficacy) at the highest dose tolerated in phase II trials.
- Phase III: This type of trial studies a prospective treatment that has shown promise. A phase III trial compares the new drug with standard-of-care therapy to evaluate its effectiveness.
- Phase IV: This phase takes place after the Food and Drug Administration (FDA) approves a new drug. Researchers evaluate long-term side effects.
How to participate in clinical trials
If you’d like to learn more about participating in clinical trials, here’s what to expect:
- Find out about clinical trial opportunities: Our doctors and research coordinators typically start the conversation about clinical trials if they think you might be eligible. You are also welcome to ask your doctors about clinical trials as an option for your treatment.
- Learn about specific trials and eligibility: Each trial has eligibility requirements specific to that trial. If you meet the requirements, we explain everything you need to know. We’ll tell you the trial’s purpose, why we are conducting the trial and which services you’ll receive.
- Complete the consent process: We discuss the risks and benefits of the trial. We also review your rights and responsibilities. For example, you have a right to privacy that includes all trials you participate in. You complete paperwork saying that you agree to participate in the trial. But you are free to change your mind at any time.
- Follow care instructions: You may need treatments at specific times or extra tests and care appointments as part of the trial. Our research coordinators help you stay on top of next steps.
Find out more about cancer clinical trials
Our clinical trials office can answer your questions regarding open clinical trials and eligibility requirements.

 Close
Close
